Think: Introduction Of A New Vaccine
| DESIGNING OF A BUSINESS ORGANIZATION | 434 |
| Introduction Of A New Vaccine | The FDA posts translations of the Pfizer-BioNTech COVID Vaccine fact sheet in multiple languages, issues a new guidance, and provides a testing update. Press Release / Public Statement 12/18/ Dec 22, · For the poorest countries in the world, Gavi, the Vaccine Alliance provides funding to assist with new vaccine introductions and has greatly accelerated the broadening of access to new vaccines Cited by: 3. 3 days ago · With Johnson & Johnson's COVID vaccines providing new optimism for local and state leaders, here in Kentucky, pharmacies will be handling the rollout of . |
| Medical Ethics Between Physician and Patient | Literacy Is The Most Important Skill We |
| Introduction Of A New Vaccine | Corporate New Ventures At Proctor And Gamble |
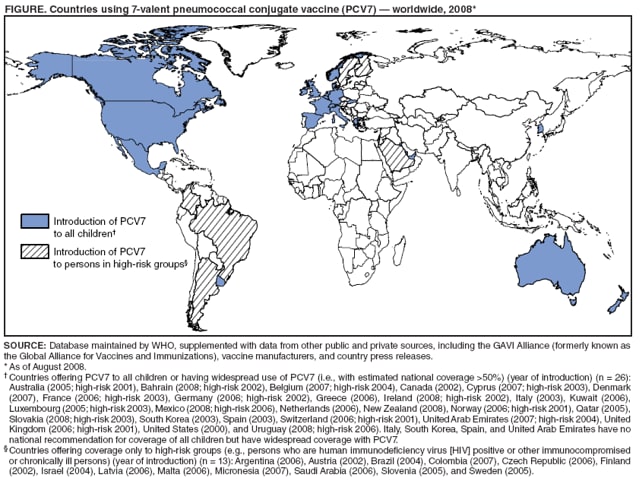
![[BKEYWORD-0-3] Introduction Of A New Vaccine](http://www.cdc.gov/mmwr/preview/mmwrhtml/figures/m6216a4f1.gif)
Introduction Of A New Vaccine Video
Vaccine : Introduction to vaccinationIntroduction
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. A Publisher Correction to this article was published on 05 January Immunization is a cornerstone of public health policy and is demonstrably highly cost-effective when used to protect child health.
Although it could be argued that immunology has not thus far contributed much to vaccine development, in that most of the vaccines we use today were developed and tested empirically, it is clear that there are major challenges ahead to develop new vaccines for difficult-to-target pathogens, for which we urgently need a better understanding of protective immunity. Moreover, recognition of the huge potential and challenges for vaccines to control disease outbreaks and protect the older population, together with Introduction Of A New Vaccine availability of an array of new technologies, make it the perfect time for immunologists to be involved in designing the next generation of powerful immunogens.
Do I really have a choice?
This Review provides an introductory overview of vaccines, immunization and related issues Vaccins thereby aims to inform a broad scientific audience about the underlying immunological concepts. Vaccines have transformed public health, particularly since read article programmes for immunization first became properly established and coordinated in the s.
In countries with high vaccine programme coverage, many of the diseases that were previously responsible for the majority of childhood deaths have essentially disappeared 1 Fig. The introduction of vaccination against infectious diseases such as diphtheria part acapsular group C meningococcus part bpolio part cHaemophilus influenzae type B part dmeasles part e and pertussis part f led to a Introduction Of A New Vaccine decrease in their incidence.

Of note, the increase in reports of H. For pertussis, a decline in vaccine coverage led to an increase in cases in the late s and s, but disease incidence reduced again after vaccine coverage increased. Adapted with permission from the Green Book, information for public Or professionals on immunisation, Public Health Englandcontains public sector information licensed under the Open Government Licence v3.

Vaccines exploit the extraordinary ability of the highly evolved human immune system to respond to, and remember, encounters with pathogen antigens. However, for much of history, vaccines have been developed through empirical research without the involvement of immunologists. There is a great need today for improved understanding of the immunological basis for click to see more to develop vaccines for hard-to-target pathogens such as Mycobacterium tuberculosisthe bacterium that causes tuberculosis TB 3 and antigenically variable pathogens such as HIV 4to control outbreaks that threaten global health security such as COVID or Ebola 56 and to work out how to revive immune responses in the ageing immune system 7 to protect the growing population of older adults from infectious diseases.
Communicating effectively about the science of vaccination to a sceptical public is a challenge for all those engaged in vaccine immunobiology but is urgently needed to realign the dialogue and Introduction Of A New Vaccine public health 8.
This can only be achieved by being transparent about what we know and do not know, and by considering the strategies to overcome our existing knowledge gaps. To achieve this, the vaccine must contain antigens that are either derived from the pathogen or produced synthetically to represent components of the pathogen. The essential component of most vaccines is one or more protein antigens that induce immune responses that provide protection.
Related to this story
However, polysaccharide antigens can also induce protective immune responses and are the basis of vaccines that have been developed to prevent several bacterial infections, such as pneumonia and meningitis caused by Streptococcus pneumoniaesince the late s 9. Protection conferred by a vaccine is measured in clinical trials that relate immune responses to the Introduction Of A New Vaccine antigen to clinical end points such as prevention of infection, a reduction in disease severity or a decreased rate of hospitalization. Schematic representation of different types of vaccine against pathogens; the text indicates against which pathogens certain vaccines are licensed and when each type of vaccine was first introduced. The distinction between live and non-live vaccines is important.
Most Popular
The former may have the potential to replicate in an uncontrolled manner Ingroduction immunocompromised individuals for example, children with some primary immunodeficiencies, or individuals with HIV infection or those receiving immunosuppressive drugsleading to some restrictions to their use By contrast, Introduction Of A New Vaccine vaccines pose no risk to immunocompromised individuals although they may not confer protection in those with B cell or combined immunodeficiency, as explained in more detail later. There is a trade-off between enough replication of the vaccine pathogen to induce a strong immune response and sufficient attenuation of the pathogen to avoid symptomatic disease.
The antigenic component of non-live vaccines can be killed whole organisms for example, whole-cell pertussis vaccine and inactivated polio vaccinepurified proteins from the organism for example, acellular pertussis vaccinerecombinant proteins for example, hepatitis B virus HBV vaccine or polysaccharides for example, the pneumococcal vaccine against S.
Toxoid vaccines for example, for tetanus and diphtheria are formaldehyde-inactivated protein toxins that have been purified from the pathogen. Non-live vaccines are often combined with an adjuvant to improve their ability to induce an immune response immunogenicity. There are only a few adjuvants that are used routinely in licensed vaccines. However, the portfolio of adjuvants is steadily expanding, with liposome-based adjuvants and oil-in-water emulsions being licensed click here the past few decades Examples of these novel adjuvants are the oil-in-water https://www.ilfiordicappero.com/custom/it-department-review-presentation/why-crimes-are-committed-by-african-americans.php MF59, which is used in some influenza vaccines 16 ; AS01which is used in one of the shingles vaccines and the licensed malaria vaccine 17 ; and AS04which is used in a vaccine against human papillomavirus HPV Vaccines contain other components that function as preservatives, emulsifiers Introduction Of A New Vaccine as polysorbate 80 or stabilizers for example, gelatine or sorbitol.
Except in the case of allergy to any of these components, there is no evidence of risk to human health from these trace components of some vaccines 19 The adaptive immune response is mediated by B cells that produce antibodies humoral immunity and by T cells cellular immunity. There is considerable supportive evidence that various types of functional antibody are important in vaccine-induced Introduction Of A New Vaccine, and this evidence comes from three main sources: immunodeficiency states, studies of passive protection and immunological data.]
Excuse for that I interfere … here recently. But this theme is very close to me. Write in PM.