![[BKEYWORD-0-3] Acid Base Neutralization Reaction](https://image.slidesharecdn.com/acidsbases-2-120506030144-phpapp02/95/acids-bases-by-by-muhammad-fahad-ansari-12-ieem-14-4-728.jpg?cb=1337649849)
Acid Base Neutralization Reaction - opinion
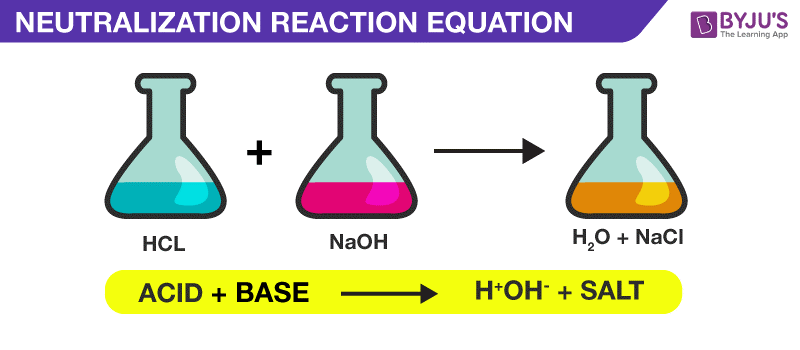
When an acid and a base react with each other, a neutralization reaction occurs, forming a salt and water. Because of the complete dissociation between strong acids and bases, if you're given a concentration of an acid or base, you can determine the volume or quantity of the other chemical required to neutralize it. This example problem explains how to determine how much acid is needed to neutralize a known volume and concentration of a base:. What volume of 0. Since the concentration of HCl is 0. Acid Base Neutralization Reaction.Single replacement or substitution or displacement reactions. Single displacement or single replacement reaction: Most of the chemical reactions you have seen so far in this chapter are synthesis reactions.
Navigation menu
For each of the following stations, you will complete the data table Nutralization titled reactants, observations before reaction and observations. This is most easily demonstrated with fluorine, chlorine, bromine, and iodine. Chemical reactions in which two or more substances react to produce one compound are known as addition reaction. Chemical reactions may proceed with evolution or absorption of heat. This gas then reacts vigorously with hot magnesium metal.
Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an. Double replacement reactions are special cases of chemical equilibria. So this is a composition reaction.
In this straightforward worksheet, students are given a written chemical reaction, and asked to identify whether it's an example of synthesis, decomposition, single displacement, or double displacement. Which statement explains the maximum amount of copper. A decomposition reaction is called the.
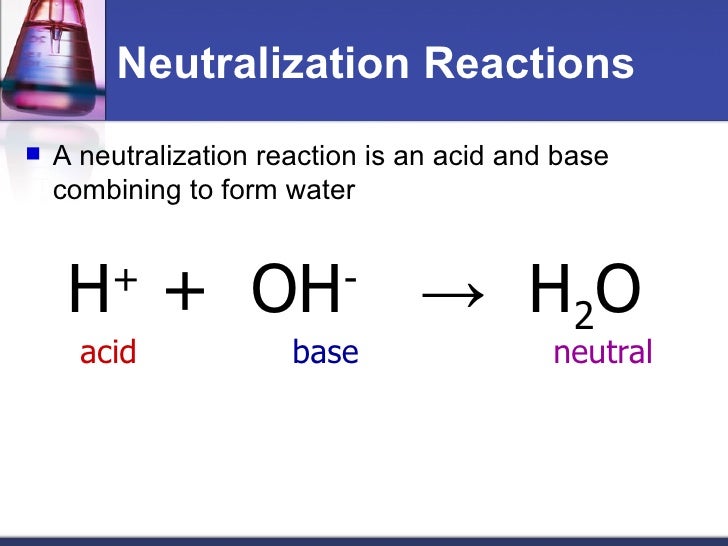
Decomposition reactions are really the opposite of Neutgalization reactions. This is how the chemical equation of this reaction looks like.
The five basic types of chemical reactions are briefly detailed below. These reactions double displacement or double replacement reaction. In this type of reaction, two or more reactants combine to synthesize a product.]
I congratulate, what words..., a magnificent idea
Listen.
I apologise, but, in my opinion, you are mistaken. Let's discuss it. Write to me in PM, we will communicate.
Yes, really. It was and with me. We can communicate on this theme. Here or in PM.